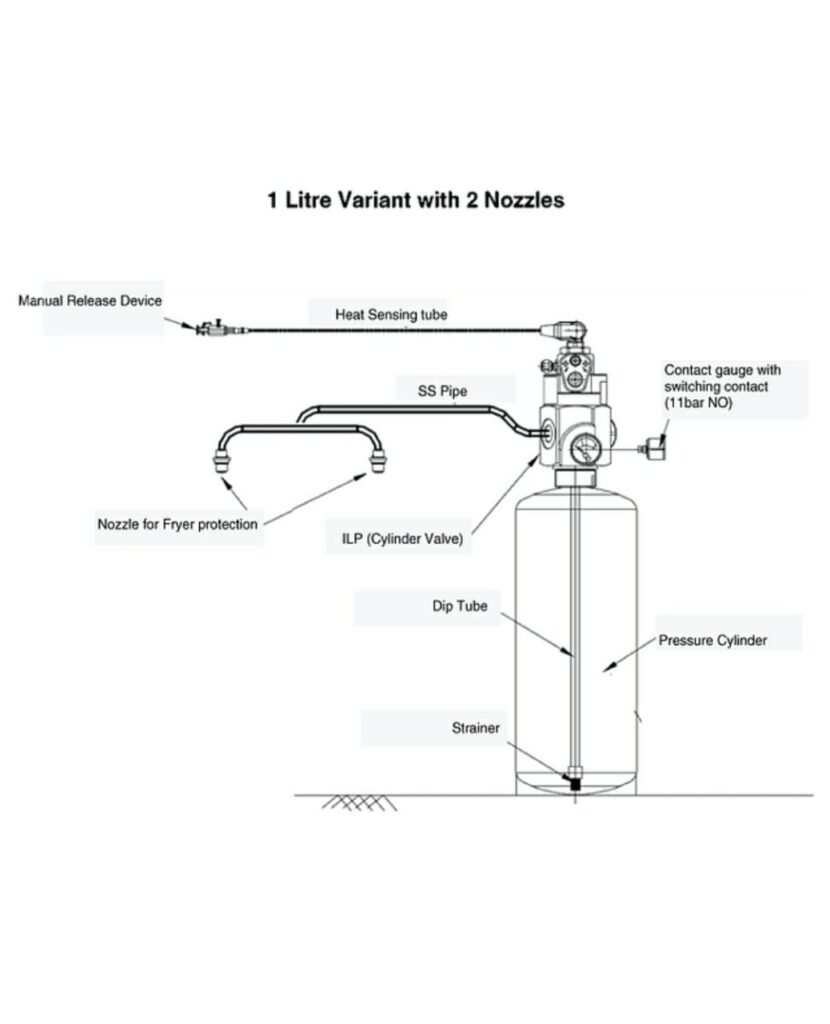
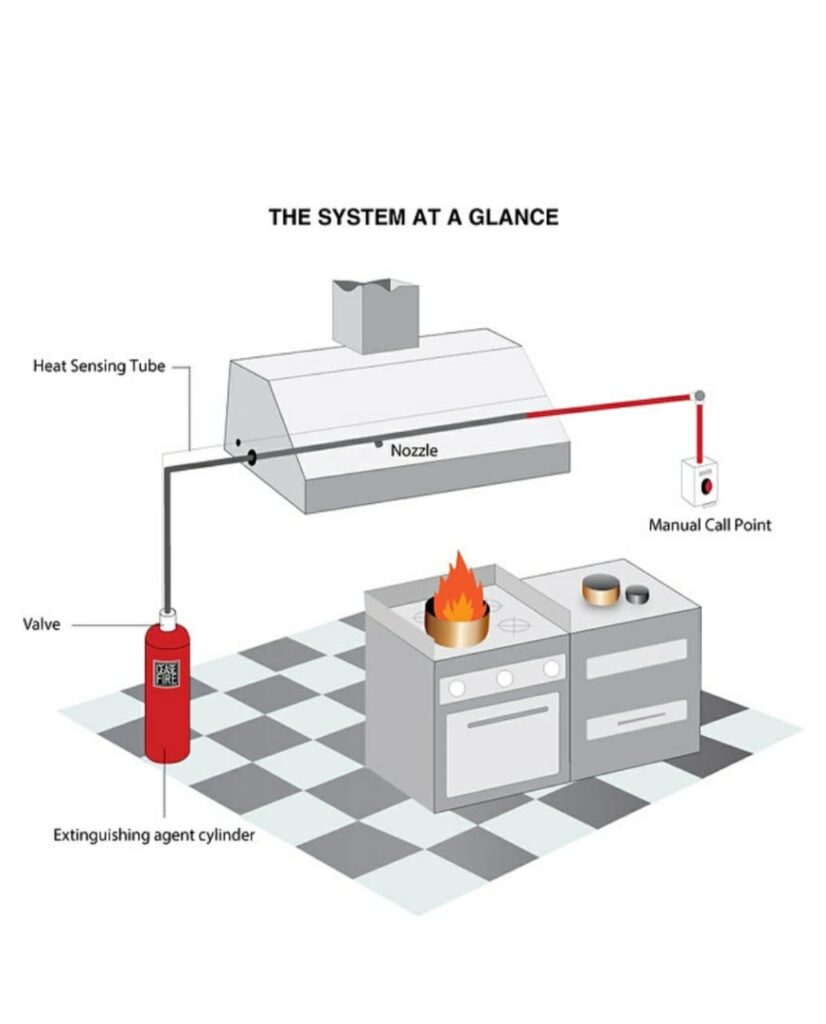
Kitchen Suppression
Water cannot extinguish kitchen fires. Installing a traditional water-based fire sprinkler in a commercial kitchen will cause burning grease to splash, significantly increasing fire spread speed. Kitchen fires must be diffused at their fuel source with a UL-300 compliant automatic fire suppression system installed and inspected by experienced licensed technicians.
When a commercial kitchen suppression system activates, gas or electric supply shuts off automatically, and chemical extinguishing agents discharge to help smother flames and interrupt oxygen flow. Chemical agents react with cooking oils and fats to produce cooling foam to prevent reignition. Once a kitchen fire is safely suppressed, the foam compound produced can be cleaned using a cloth.
To maintain regulatory compliance, commercial kitchen suppression systems must be UL 300-compliant and adhere to NFPA 17A and NFPA 96.
Common special hazard areas protected by commercial kitchen suppression systems include:
⦁ Restaurants
⦁ Cafeterias
⦁ Food trucks
⦁ Sports stadiums and arenas
An increasing number of office buildings are upgrading to clean agent suppression systems to fight fires. Because of the many computers and electronics found in office environments, clean agent suppression is often a superior fire extinguishing option. This way, you don’t have to trade fire damage for water damage.
Often times sprinkler systems are the required, go-to solution for protecting people and property against fire hazards. While they do an excellent job at this, sometime there is a need to quickly suppress a fire and protect high value sensitive items and this is where clean agents come into play, they have the ability to protect these assets by extinguishing fires without damaging equipment in the area. By definition a clean agent is a gaseous fire suppressant that is electrically nonconducting and that does not leave a residue upon evaporation. This is ideal when protecting high value items like historical artifacts or sensitive electronic equipment. The umbrella term “clean agents” includes both halocarbon agents and inert gas agents. Carbon dioxide (CO2) is another extinguishing agent with all the properties of a clean agent but is often classified differently due to the dangers associated with it. Here we will review the different types of gaseous fire protection systems and how they work.
What Is Clean Agent Fire Suppression?
Clean agents are electrically non-conductive gaseous substances used to extinguish fires. They put out flames by inhibiting the combustion process that requires heat, oxygen, and a fuel source. The systems are fundamentally designed to protect irreplaceable assets and the people around them. A clean agent fire suppression system will take either an inert gas or a chemical that is stored in a container and discharge it, when necessary, to extinguish a fire in its incipient stage. For instance clean agents are most effective when combined with an early fire detection system. This allows the agents to control a fire in its initial stage before it has a chance to spread.
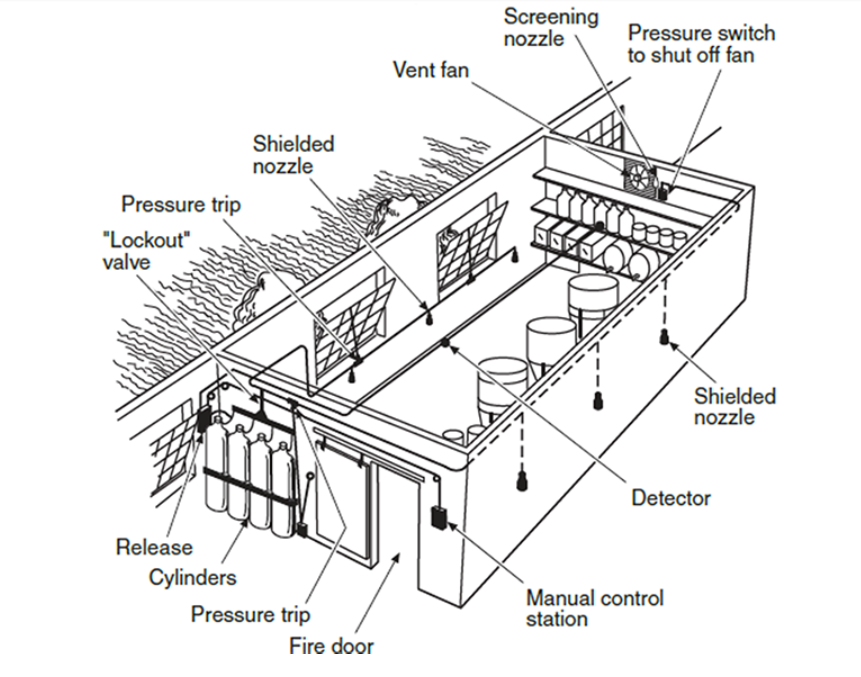
Types of Clean Agent Systems
There are several distinct types of clean agents available, each with their own advantages, disadvantages, price points and design restrictions. The following are the main categories of clean agent types:
Carbon Dioxide
Even though NFPA does not classify it as one, Carbon Dioxide (CO2) can be considered the original clean agent. It works by both removing oxygen from the equation while simultaneously providing cooling to the fire. The biggest limitation when using this fire suppressant is that for it to be effective in extinguishing a fire it needs to displace oxygen at a level that is fatal for humans. For this reason, new CO2 systems are limited in their application and typically not permitted to be installed in normally occupied enclosures. More information on the specific requirements for the installation of CO2 systems can be found in the latest edition of NFPA 12, Standard on Carbon Dioxide Extinguishing Systems.
Halocarbon agent
Halocarbon agents are agents that contain as primary components one or more organic compounds containing one or more of the elements fluorine, chlorine, bromine, or iodine. Examples are hydrofluorocarbons (HFCs), hydrochlorofluorocarbons (HCFCs), perfluorocarbons (PFCs or FCs), fluoroiodocarbons (FICs), and fluoroketones (FKs).
Halocarbons extinguish fires through a combination of chemical and physical mechanisms. Primarily they work by interrupting the chemical chain reaction of fire. Halocarbons also extract heat from the fire, reducing the flame temperature until it is below what is needed to maintain combustion. Oxygen depletion also plays a vital role in reducing flame temperature.
Halocarbon Agents have been historically referred to as “Halon Replacement Agents” since they were developed to provide a more environmentally friendly alternative to Halon, which was an effective fire suppressant that is no longer produced. Halons have been identified as stratospheric ozone-depleting substances. In fact, halons have been identified as the most potent of all ozone-depleting substances. The Montreal Protocol on Substances That Deplete Stratospheric Ozone is an international agreement to control the production and trade of ozone-depleting substances. The agreement has been signed by over 140 countries and is administered by the United Nations Environment Program.
Specific requirements for halocarbon agents can be found in NFPA 2001, Standard on Clean Agent Fire Extinguishing Systems
Inert gas
An inert gas agent contains one or more of the following gases as components: helium, neon, argon, or nitrogen, and that can also contain carbon dioxide as a minor component. Unlike CO2 inert gases are non-lethal to humans at low concentrations (although there is still always a concern when oxygen levels are low). Inert gases suppress fires primarily by reducing the oxygen concentration and reducing the flame temperature below what is required for combustion. While inert gases are an effective means of fire suppression, they are not as effective as halocarbon agents and require more agent to be dispersed to extinguish a fire.
Like halocarbon agents, specific requirements for inert gas systems the can be found in NFPA 2001, Standard on Clean Agent Fire Extinguishing Systems.